Organization
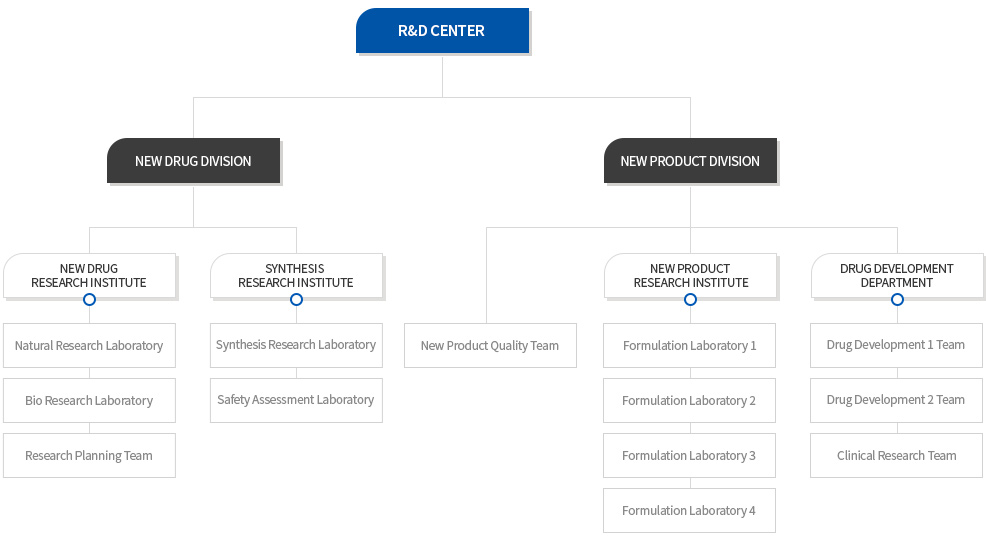
NEW DRUG DIVISION
New Drug Research Institute
| Natural Research Laboratory |
1) Item excavating and development of therapy from natural products 2) Study of manufacturing process optimization and standardization 3) Development of standards and methods |
|---|---|
| Bio Research Laboratory |
1) Evaluation of the efficacy of candidates 2) Formulation study of candidates 3) Pharmacological mechanism research 4) Development of stem cell therapeutics |
| Research Planning Team |
1) Research planning for the discovery of new drug candidates, and confirmation of research output 2) Application and management of government-supporting R&D project 3) Finding joint research projects and building the cooperating network, industry-academy-research institute-hospital |
Synthesis Research Institute
| Synthesis Research Laboratory |
1) Synthesis research for candidates of new drugs 2) Process development of generic APIs 3) Process optimization and pilot scale production 4) Troubleshooting of commercial production 5) Synthesis of impurity standards |
|---|---|
| Safety Assessment Laboratory |
1) Impurity profiling study including genotoxic and elemental impurities 2) Stability study of intermediates and APIs 3) Development of new analytical methods 4) CTD documentation 5) Analysis support for synthesis research |
NEW PRODUCT DIVISION
New Product Quality Team
| New Product Quality Team |
1) Preparing data and supplementary data for NDA(New Drug Application) 2) Validation and quality management of new products 3) Management of investigational products 4) Management of schedule and progress of formulation improvement |
|---|
New Product Research Institute
| Formulation Laboratory 1 Formulation Laboratory 2 Formulation Laboratory 3 Formulation Laboratory 4 |
1) Formulation Research
|
|---|---|
2) Analytical Research
|
Drug Development Department
| Drug Development 1 Team Drug Development 2 Team |
1) Derivation of development items, establishment of development strategies and management of development schedules 2) In/Out licensing 3) Application and registration management of patents/trademarks, responding to patent litigation 4) Design of clinical trials and conduct of bioequivalence trials 5) CTD documentation and documentation required for marketing authorization/registration 6) NDA(New Drug Application) and DMF(Drug Master File) registration 7) Renewal and re-evaluation of drug license |
|---|---|
| Clinical Research Team |
1) Development of clinical trial-related documents 2) IRB(Institutional Review Board) registration and responding to IRB 3) Conduct of phase Ⅰ, Ⅱ, Ⅲ and Ⅳ clinical trials 4) Conduct of PMS(Post Marketing Surveillance) and SIT(Sponsor-Investigator Trials) 5) Clinical trial-related CTD documentation 6) Design of investigational products packaging 7) Responding to inspection from MFDS(Ministry of Food and Drug Safety) |